Patient : AV Graft Disease
According to the National Kidney Foundation, about 20 million Americans are currently affected by kidney and urological diseases and millions more are at risk. In the U.S., approximately 300,000 patients suffer from End-Stage Renal Disease (ESRD); 85% of these patients will be treated by hemodialysis. In the absence of a kidney transplant these patients will require artificial hemodialysis for their lifetime. Diabetes Mellitus (Type II adult onset) is the leading cause of chronic kidney failure, accounting for 35% of the new cases each year. Uncontrolled high blood pressure is the second leading cause of chronic kidney failure in the U.S., resulting in approximately 30% of the new cases each year.
Hemodialysis requires a direct artery-vein access that is easy to locate and that will provide optimal blood flow during treatment. Unfortunately, a number of hemodialysis patients do no have adequate vessels to form a fistula (a connection) between a vein and an artery; therefore, a synthetic graft must be inserted. Synthetic grafts are the most widely used type of vascular access in the U.S., the most common being the polytetrafluorethylene (PTFE) graft. PTFE grafts are easily inserted, easily maintained, have moderate resistance to infection and a low incidence of aneurysm formation. Typically, these grafts are placed as a straight connection or as a loop in either the forearm or arm; this loop connects an artery to a vein and allows for easy access during hemodialysis treatment.
As reported by the United States Renal Data System (USRDS), hemodialysis access failure is the most frequent cause of hospitalization among ESRD patients, and costs the U.S. healthcare system approximately 1 billion dollars per year. Preservation of access sites is of paramount importance as patients are being treated with hemodialysis for longer periods (almost 50% are treated for 5 years). Balloon angioplasty has been used for maintaining, rather than restoring, the graft when tissue growth narrows the passageway. Though surgery is reasonably effective, there are associated problems. Balloon angioplasty is usually required over the long term, since uncontrolled tissue growth within the graft is very common. The long-term follow-up information on PTFE grafts show that 60% have narrowed within the first year, and 40% have arrowed by 2 years.
One potential solution for patients with a hemodialysis graft stenosis is enrollment into the BRAVO Trial (Beta Radiation for Treatment of Arterial - Venous Graft Outflow). The BRAVO Trial uses catheter-based vascular brachytherapy to temporarily deliver radiation to the site of tissue overgrowth. The mechanism by which radiation therapy affects tissue growth is thought to be on a cellular level, stopping the growth of new cells. Over the past three years, vascular brachytherapy has become an accepted method for the treatment of uncontrolled tissue growth within stented heart vessels. Though the information concerning use of radiation in the treatment of hemodialysis grafts is preliminary, the basic process underlying graft failure is one of uncontrolled tissue growth. Scientifically, there is little to distinguish the uncontrolled tissue growth with a hemodialysis graft from the uncontrolled tissue growth seen in the coronary or peripheral arteries; the only difference lies in the initial cause.
The BRAVO Trial has been developed to study the safety and effectiveness of our Strontium90-based peripheral vascular brachytherapy system, for the treatment of venous outflow stenosis in patients with hemodialysis grafts. Patients will be randomized to treatment with vascular brachytherapy or a placebo procedure following their angioplasty procedure.
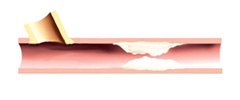
1. Access flow screening will be completed to determine trial eligibility.
2. Angiographic screening will be completed to confirm inclusion into the trial.
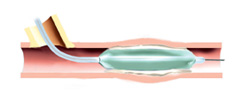
3. Patient will have balloon angioplasty procedure.
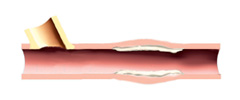
4. Successful angioplasty procedure will be confirmed.
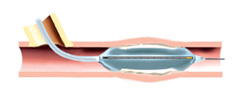
5. Patient will have vascular brachytherapy treatment or a placebo procedure.
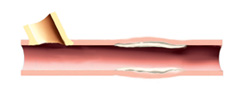
6. Six month angiographic follow-up will confirm graft patency.
For information regarding hospitals and physicians in your area that participate in the BRAVO Trial, please call 800-NOVOSTE.